Adventitial Delivery of Agents for the Treatment and Prevention of Restenosis
FEATURES Balloon angioplasty is one of the most widely used treatments for heart and vascular disease with an estimate of nearly two million procedures performed each year. However, roughly 25% of patients treated with balloon angioplasty experience renewed narrowing of the treated arteries, or restenosis, within six months of the initial procedure, often leading to further interventions. Indiana University researchers have developed a broad method for the non-intramural, adventitial (outside lining) delivery of biologically active substances that, in its most obvious use, may obviate the need for a drug eluting stent and potentially eliminate the need for a stent in conjunction with the treatment or prevention of restenosis. This method entails localized in vivo delivery of a substance over time through the administration of any biologically active substance using specific carriers administered to the non-intramural adventitia (outside surface) of the area to be treated e.g. the percutaneous delivery of a heparin containing gel or polymer to the outside surface of a vessel that recently underwent angioplasty.BENEFITS
* Minimally invasive
* Time released delivery of therapeutic agents to vessels, organs, or other internal areas.
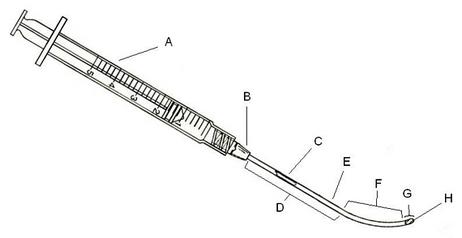
Fig 1: Side elevation view of one embodiment of this invention: a slender tubular device shown in conjunction with a syringe, having a cut-out cross-sectional view depicted therein. The individual sections include a syringe (A), a hub (B) to connect the needle (E), a lumen (C) in the needle, a linear section (D) and a curved section (F) of the needle, a lumen opening (G), and a piercing part of the needle itself (H).
Attached files:
Patents:
US 5,893,839
US 6,200,302
Inventor(s): Matthew S. Johnson, M.D.
Type of Offer: Licensing
« More Medical Devices Patents
