Novel Catalyst Exchanges Deuterium or Tritium into Organic and Organometallic Compounds
APPLICATION OF TECHNOLOGY:Preparing deuterium- or tritium-labeled organic and organometallic compounds for:
- Manufacturing pharmaceuticals, chemicals, and agricultural products - Spectroscopic experiments - Research on chemical structure and reaction mechanisms - Analytical tracers ADVANTAGES:
- Catalyzes hydrogen/deuterium (H/D) exchange from the least expensive deuterium and tritium source, heavy water, into organic and organometallic compounds with activated or unactivated protons Enables the profitable production of new commercial deuterium- and tritium-labeled compounds and will make others less expensive to prepare - Employs a catalyst that is easy to remove from products, is stable in air, and is moderately stable in water - Unlike processes using platinum-based catalysts, strongly acidic conditions are not necessary, allowing exchange into organic compounds that would destabilize in the presence of an acid - Catalytic activity is higher than that of previously reported rhodium and iridium complexes - The H/D exchange occurs under conditions that are inexpensive and easy to achieve: heating temperatures of between 70 and 150 degrees C and atmospheric pressure
ABSTRACT: Robert Bergman and Steven Klei have discovered a class of transition metal compounds that catalyze the exchange of deuterium from heavy water (D2O) into a wide range of organic and organometallic compounds that are otherwise difficult to deuterate. The process is ideal for commercialization for several reasons. It uses the least expensive source of deuterium, heavy water, for the exchange, overcoming the fact that water is normally non-reactive with organic compounds, and when it is reactive, it typically participates in H/D exchange only with activated protons. The Berkeley Lab catalyst causes heavy water to exchange with both activated and unactivated protons. In addition, while some other isotope labeling processes require very high temperatures and pressures, this exchange occurs under moderate temperatures and atmospheric pressure, minimizing processing costs. Furthermore, the most active catalyst discovered by Bergman and Klei is air stable and can be prepared in two high-yielding steps from commercially available compounds. This breakthrough promises to make deuterium- and tritium-labeled organic and organometallic materials less costly and more readily available.
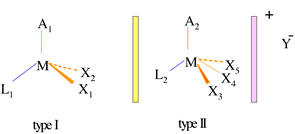
The general structure of the two types of transition metal complexes serving as catalysts in this process are delineated in the figure above where, in the ionic model, A1 and A2 represent any ligand that can donate six electrons, L1 and L2 represent charge neutral two-electron donors, X1 , X2 , X3 , X4 , and X5 stand for two-electron donors, M is a group VIII metal, and Y is a negatively charged anion capable of creating a charge-neutral complex. The starting materials then, are 18 electron species that are assumed to form a 16 electron catalytically active species, by either dissociating a ligand (X1 or X2), i.e. ionizing, in the case of type I, or reductively eliminating a small molecule (X3, X4 or X5) in the case of type II.
Catalysts of the types described above can be prepared by known methods. When the compound is contacted with a source of deuterium and a substrate molecule, and the combination is heated at a temperature between 75 and 150 degrees Celsius, one or more deuterium and/or tritium atoms exchanges with one or more protons of the organic substrate molecule. The now deuterium-tagged organic molecule can be separated or isolated by readily available techniques.
Attached files:
Patents:
US 6,794,522
Inventor(s): Robert Bergman and Steven Klei
Type of Offer: Licensing
« More Medical Patents
