A Method of Predicting Response to Thalidomide in Multiple Myeloma Patients
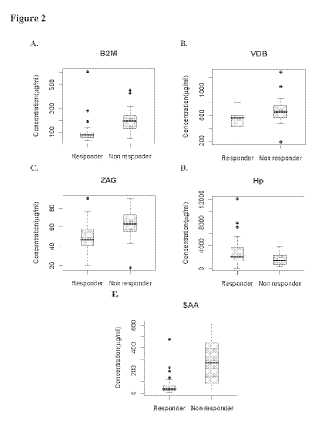
A method of predicting response to thalidomide, or thalidomide analogs, in an individual with cancer, especially cancers for which thalidomide has been implicated as a treatment, such as Multiple Myeloma (MM) employs one or more of a panel of biomarkers that have been shown to be differentially expressed in cancer patients that respond to thalidomide (hereafter "Responders") relative to cancer patients that do not respond to thalidomide (hereafter "Non-responders). The method involves assaying a biological sample from the individual to determine the abundance of at least three biomarkers including Vitamin-D binding protein precursor(VDB) (Sequence ID 1) and Serum amyloid A protein (SAA) (Sequence ID 3), and at least one of beta-2-microglobulin (B2M) (Sequence ID 4), Haptoglobin (Hp) precursor (fragment) (Sequence ID 5), and zinc-alpha-2- glycoprotein (ZAG) (Sequence ID 2). Correlation of the abundance value for the at least three biomarkers with a reference abundance value from a Responder or Non-responder enables predication of response to thalidomide for the patient.Attached files:
Patents:
WO 2,011,020,839
Inventor(s): RAJPAL RAJESH [IE]; DOWLING PAUL [IE]; CLYNES MARTIN M [IE]; O'GORMAN PETER [IE]; CLARKE COLIN [IE]
Type of Offer: Sale
« More Medical Patents
